Graham's DIffusion Law
Graham's DIffusion Law: Overview
This topic covers concepts, such as, Diffusion of Gases, Graham's DIffusion Law, Effusion of Gases & Applications of Graham's Diffusion Law etc.
Important Questions on Graham's DIffusion Law
Two gases A and B having the same volume diffuse through a porous partition in 20 and 10 seconds respectively. The molecular mass of A is 49 u. Molecular mass of B will be:
From a heated mixture of nitrogen, oxygen and carbon, two compounds (out of the many obtained) are isolated. The rates of diffusion of the two isolated compounds are almost identical. The two compounds are
Pure diffuses through an aperture in where as mixture of and another gas containing diffuses from the same in What is molecular weight of the gas.
A gas mixture contains equal number of molecules of and some of it is passed through a gaseous effusion apparatus. Calculate how many molecules of are present in the product gas for every molecules of
From a certain apparatus, the diffusion rate of hydrogen has an average value of . The diffusion of another gas under the same conditions is measured to have an average rate of . Identify the gas.
From a certain apparatus, the diffusion rate of hydrogen has an average value of . The diffusion of another gas under the same conditions is measured to have an average rate of . Identify the gas.(Oxygen/Nitrogen)
The degree of dissociation of the reaction in terms of rate of diffusion of equilibrium mixture and that of a reference gas is given by:
where stands for the respective molar masses.
In , the volume of gas effuses through a hole in a container. The time taken for the effusion of the same volume of the specified gas under identical conditions is
at and at pressure are effused through a small orifice under similar conditions, ratio of their rate of effusion is -
Molar ratio in which and should be mixed so that when mixture is allowed to effuse through pinhole, initially both gases comes out at equal rate?
of each gas and of gas takes and seconds respectively for effusing through a pin hole under the similar conditions. If molecular mass of gas is , the molecular mass of gas will be :
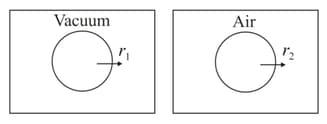
A gas is effusing in vacuum at rate and in air at rate , then :
Calculate relative rate of effusion of to through a container containing and in mass ratio :-
The rate of diffusion of at a given temperature is twice that of a gas . The molecular weight of is :-
At constant pressure and temperature the rates of diffusion and of gases and having densities and are related as :-
The molecular weight of a gas which diffuses at of the speed of hydrogen under identical conditions is :-
Which pair of the gaseous species diffuse through a small jet with the same rate of diffusion at same and :-
If the rate of diffusion of unknown gas is twice that of methane gas under identical condition of temperature and pressure, the molar mass of the unknown gas is
Four gas balloons of equal volumes containing respectively were pricked with needle and immersed in a tank containing at same pressure which of them will not shrink after some time :
A certain gas diffuses from two different vessels and . The vessel A has a circular orifice and vessel has square orifice and radius of the orifice of vessel is equal to side of orifice of vessel . The ratio of rate of diffusion of gas from vessel to vessel , assuming same temp and pressure is.
